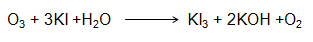
Principle: Micro-amounts of Ozone (O3) collected by absorption in a solution of potassium iodide buffered to a pH of 6.8.The released iodine equivalent of the concentration of ozone present in the air is determined by measuring the absorption of tri-iodide ion at 352nm.
Interference Reducing gases such as Sulfur dioxide is a major interferent. Its interference can be removed by using Chromium trioxide trap.
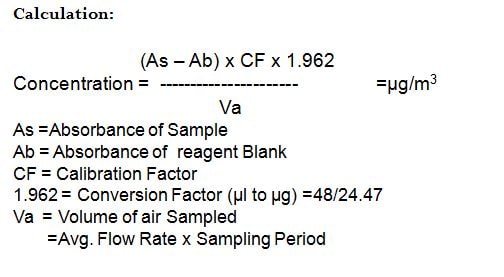
Range & Sensitivity: The range of the method is 19.6 – 19,620ug/m3. (0.01ppm to 10ppm).
(When 10ml of absorbing solution is used, between 1 to10µl of ozone(1.962 to 19.62µg) corresponds to absorbance between 0.1 and 1 in 1cm cell are collected)
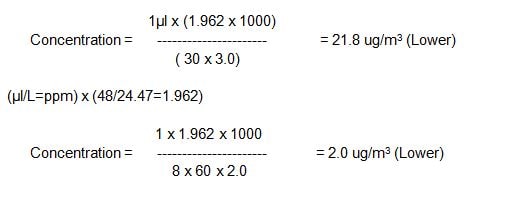
Preparation of SO2 Trap:
step 1: Take GF/A 8X10” Filter Paper
step 2: Drop 15ml aqueous solution of 2.5gm chromium trioxide mixed with 0.7ml Conc.H2SO4
step 3: Dry Filter Paper in oven at 90oC for 1 hour
step 4: Quickly cut the pieces of filter paper and stored in airtight bottle
step 5: Fill these filters in trap of impinger just before sampling
Preparation of Reagent
A. For Sampling:
Absorbing Reagent (1%KI in 0.1M Phosphate Buffer) : Dissolve 14 gm of Pot. dihydrogen Phosphate (kH2PO4) , 14.2gm of disodium hydrogen phosphate (Na2HPO2) and 10.0gm of potassium iodide in sequence and dilute the mixture to 1litre with distilled water.
Keep at room temperature for one day.
Measure pH and adjust to 6.8±0.2 with NaOH or KH2PO4.
B. For Analysis: No Solution required
C. For Calibration Curve:
1. Stock Solution 0.025MI2(0.05N): Dissolve 16gm of Potassium Iodide and 3.173gm of resublimed iodine and dilute the mixture to 500ml with distilled water. Standardized against 0.025M Sodium Thiosulphate.
2. Standard Solution 0.001MI2: Dilute 4.0ml of 0.025M stock solution with absorbing reagent up to 100ml. (Caution :Protect from Sun Light)
3. Working Standard Solution (1µl O3/ml): Dilute 5.11ml of 0.001M I2 Solution with absorbing reagent up to 100ml.
Sampling:
step 1: Place 10 ml of absorbing reagent in an impinger
step 2: Sample for desired time at flow rate 1.0 lpm for 1 hour
step 3: After Sampling measure the volume of absorbing reagent
step 4: Makeup the evaporating absorbing reagent with distilled water as initial volume
Preparation of Calibration Curve (O3)
1.Pipette 0.0 (blank), 1.0, 2.0, 4.0, 6.0 , 8.0 and 10.0 ml of 1.0µl of O3 / ml standard solution in a series of 10ml glass stopper graduated cylinder.
2.Make up to 10ml with absorbing reagent and mix thoroughly.
3.Maintain all the solutions and sample at 25 oC(Room temp.)
4.Measure the absorbance at 352nm against the field blank reagent.
5.Plot a curve absorbance (Y-axis) verse concentration (X-axis) .